The development strategy focuses on providing patients with in-demand vaccines and biotech drugs for the prevention and treatment of infectious, orphan, and oncological diseases

The product portfolio includes original pharmaceutical products, contract medicines and food supplements

R&D center, patents for molecules and pharmaceutical manufacturing technologies in Russia and abroad

Modern biotechnological production in accordance with the EAEU and EU GMP standards

One of the largest exporters of original pharmaceutical products and vaccines for the prevention of influenza

Partner of international Big Pharma companies: Pfizer, Abbott, Boehringer Ingelheim, ISU ABXIS
Structure of "Petrovax"
-
R&D center
-
Biotech portfolio: BD, market access, technology transfer
-
Clinical studies and product registration
-
Administration office
-
Synthesis of APIs, manufacturing of finished-dosage forms
-
Quality Control
-
Retail portfolio: marketing, sales force, commerce, BI, BD, medical department, export
-
Biotechnological drugs and vaccines: marketing, sales budget, export
-
Pharmacovigilance
The company was founded in 1996 by a team of Russian scientists.
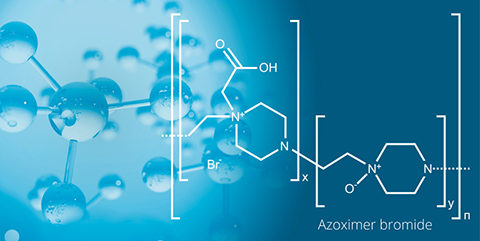
Leading pharmaceutical innovations
Expanding access to cutting-edge therapies
 Company presentation
(PDF 6 МB)
Company presentation
(PDF 6 МB)
















